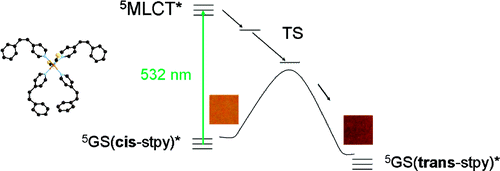
We report a thorough investigation of the absorption spectra of the cis and trans isomers of the 4-styrylpyridine photoswitch based on TDDFT calculations. The spectra of both isomers were analysed first from the results of excitation calculations performed on their optimised geometries. The main absorption band of the cis isomer is thus predicted to be due to the S0 ‚Üí S1 and S0 ‚Üí S2 transitions, while the main absorption band of the trans isomer is predicted to originate exclusively from the S0 ‚Üí S1 transition. The convolution of the calculated oscillator strengths with Gaussians helped mimic the broadening of the electronic transitions. However, it proved necessary to use Gaussians with a large full width at half maximum of 5000 cm-1; and, compared to experiment, the calculated main absorption bands of the two isomers are significantly red-shifted and far too symmetric. Consequently, as required for the detailed analysis of the finite-temperature absorption spectrum of a molecule as flexible as 4-styrylpyridine, the influence of the thermal fluctuations has been taken into account by calculating the spectra as time averages over Car‚ÄďParrinello molecular dynamics trajectories. For both isomers, this led to a noticeable improvement in the relative positions of the calculated and experimental main absorption bands, and the asymmetry of the calculated bands brings them in better agreement with the experimental ones. Furthermore, these last results show that, actually, the S0 ‚Üí S1 and S0 ‚Üí S2 transitions both contribute significantly to the finite-temperature main absorption bands of the two isomers. Finally, in order to also take the vibrational broadening into account, the Franck‚ÄďCondon factors of the relevant vibrations were calculated within the displaced harmonic oscillator approximation. By thus taking both the thermal and the vibrational broadening into account for the calculation of the absorption bands, the agreement between experiment and theory could be further improved. |